
Clinical trial using umbilical cord stem cells for treating severe COVID-19 shows promising results
Successful clinical trial calls for additional larger trials to examine the use of mesenchymal stems cells to improve survival rates and recovery time
On January 20, 2020, the US reported its first case of COVID-19. In the ensuing year, the virus affected more than 24 million people leading to over 400,000 death in the US alone. As we hope to return to ‘normalcy’ with the advent of the vaccines, find more effective methods for treating those affected with COVID-19 remains equally important.
Here we summarize results from one successful clinical trial, which shows umbilical-cord derived stem cells as a safe and effective option for improving the survival rate and recovery time of those with severe COVID-19.
 The experiment
The experiment
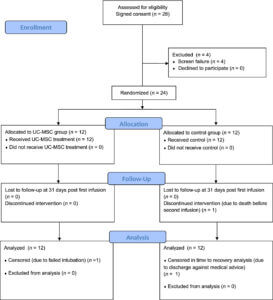
This small clinical trial involved 24 subjects with COVID-19 acute respiratory distress syndrome or ARDS. Patients with ARDS experience severe respiratory symptoms, which means the study looked at the sickest COVID-19 patients.
The trial was designed to be double-blind, randomized, and controlled, which is the gold standard for conducting clinical trials. Double blinding ensures that neither the patient nor the researcher knows who receives the treatment and who receives the placebo. Randomization assigns patients into either a treatment group or a control group, so the results can be compared.
The treatment group received umbilical cord-derived mesenchymal stem cell infusions on day 0 and day 3. The control group received a control solution without stem cells. You can find out more about umbilical cord stem cells here. Both groups received the best standard of care following the treatment.
 The results
The results
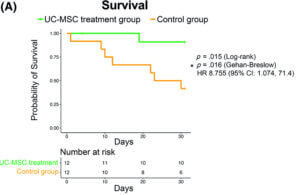
First, to determine the treatment was safe, patients were monitored for up to 24 hours post-treatment. There was no difference in the number of adverse events (defined as cardiac arrest or death following treatment) between the control group and the treatment group, indicating that stem cell infusion was safe.
When they looked at the survival rate one month after receiving the first infusion, 91% of the patients who received the stem cells were still alive compared to only 42% of the patients in the control group. These results suggest that patients who do not receive treatment are at higher risk of death from severe COVID-19.
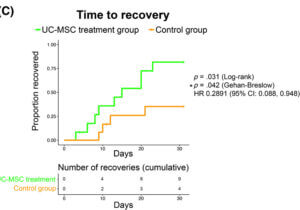
Finally, patients who received stem cells recovered faster than the control group where 80% of patients in the treatment group recovered by day 30 compared to the less than 37% rate of recovery in the control group.
 The conclusion
The conclusion
The researchers started on this trial to establish that using umbilical stem cells to treat severe cases of COVID-19 was safe. Not only did the findings support this claim, but they also found that patients who received two doses of stem cells were more likely to survive and recover. These promising observations now call for a much larger study so that umbilical stem cells can become a widespread treatment option for severe cases of COVID-19.
Reference
Lanzoni, G, Linetsky, E, Correa, D, et al. Umbilical cord mesenchymal stem cells for COVID‐19 acute respiratory distress syndrome: A double‐blind, phase 1/2a, randomized controlled trial. STEM CELLS Transl Med. 2021; 1– 14. https://doi.org/10.1002/sctm.20-0472


The Hospital Bag Checklist

Sibling cord blood tested for treating cerebral palsy in young children

Categories
Related Posts

Top 10 items to include in your baby registry

The Hospital Bag Checklist

Sibling cord blood tested for treating cerebral palsy in young children





